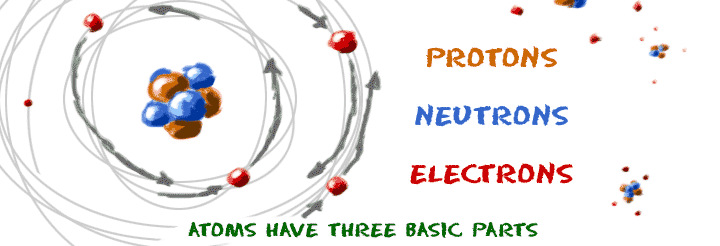
Atoms Protons Electrons
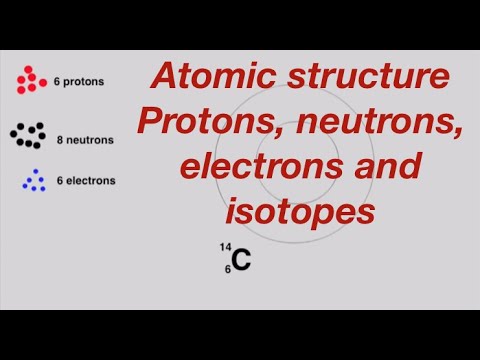
For example carbon 12 carbon 13 and carbon 14 are three isotopes of carbon each having 6 electrons.
Atoms protons electrons. Electrons are particles that have a negative charge equal to 1. Electrons are extremely lightweight and exist in a cloud orbiting the nucleus. Protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. Determine the number of protons neutrons and electrons in an atom.
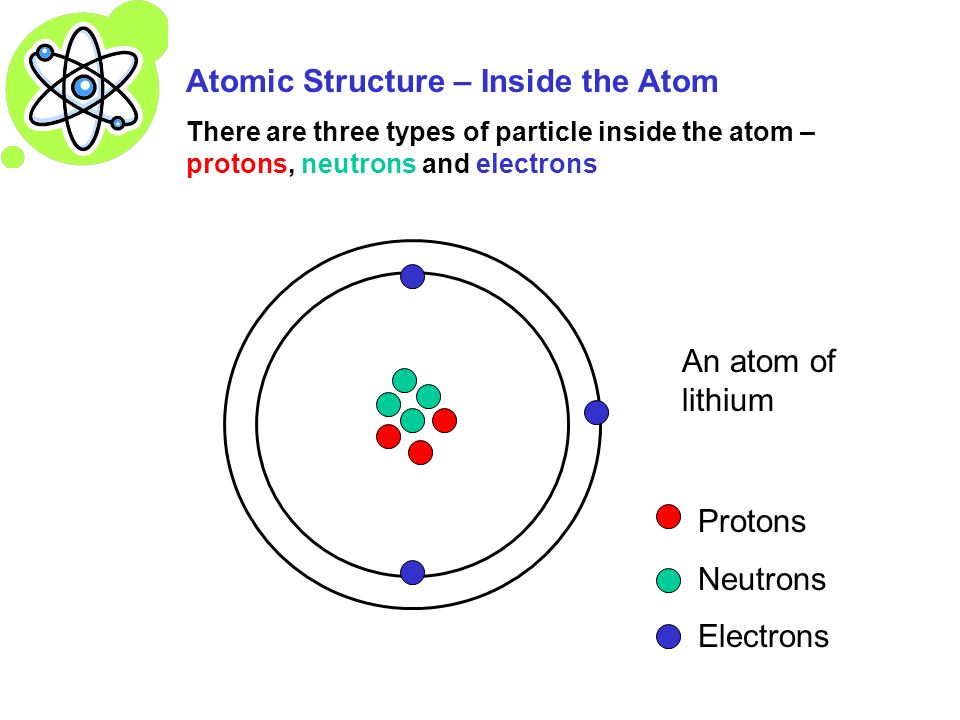
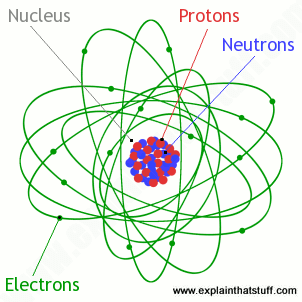
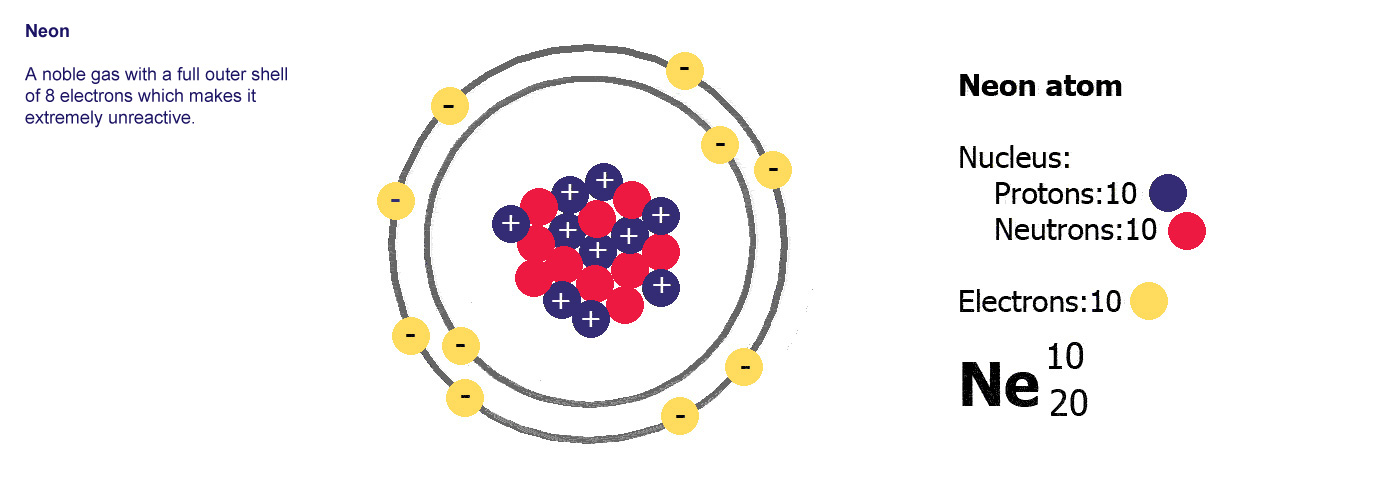
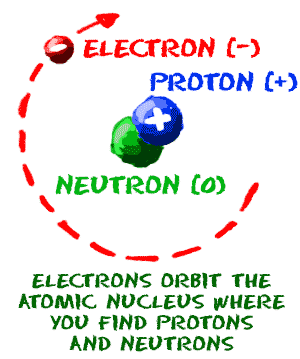
A neutral atom has the same number of protons and electrons charges cancel each other out. Their total negative electrical charge is equal to the protons positive electrical charge. The small and dense nucleus hold the protons and the neutrons. Electrons surround the nucleus.
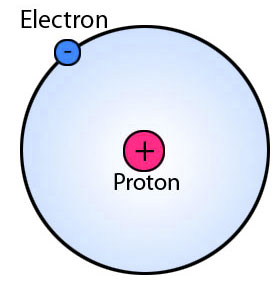
If a neutral atom has 1 proton it must have 1 electron. Protons and neutrons are in the center of the atom making up the nucleus. The electrons are found in the electron cloud. You get the idea.
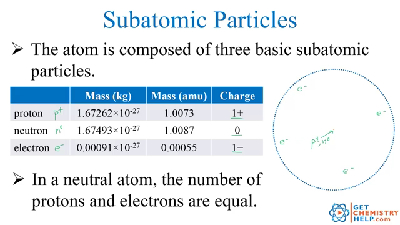
In order to be neutral an atom must have the same number of electrons and protons. The charge on the proton and electron are exactly the same size but opposite. Atoms are made of protons neutrons and electrons. If a neutral atom has 10 protons it must have 10 electrons.
Electrons circle around the nucleus and have a negative electrical charge. Protons have a positive charge. An ion has an unequal number of protons and electrons. Protons carry a positive electrical change while electrons are negatively charged and neutrons are neutral.
Determine the number of electrons. Finding protons neutrons and electrons of isotopes isotopes are atoms of the same element with the same proton number but different number of neutrons. This area takes up most of the space of the atom. Therefore an element in a neutral state will have the same number of protons and electrons.
Atoms always have the same number of electrons protons and neutrons. Electrons have a negative charge. Atoms are made of extremely tiny particles called protons neutrons and electrons. Protons are particles in the nucleus of an atom that have a positive charge equal to 1.












:max_bytes(150000):strip_icc()/GettyImages-523446050-5897be0a5f9b5874ee7c9fa6.jpg)
